As we have seen in recent weeks as Covid-19 and Hydrochloroquine cocktail treatments have been on the table, there is a clear tendency to view and treat double-blind placebo controlled testing as a “gold standard” yardstick and to then use such to discount and dismiss whatever does not “measure up” such as Professor Raoult’s work over in France. I will now argue in outline that such an attitude is selectively hyperskeptical, seriously ethically, epistemologically and logically flawed, and sets up a crooked yardstick.
It is a commonplace in Medical research that arguably more lives were saved, net, than perished through the tainted medical studies in the Nazi death camps. However, the taint was seen as so serious that a programme of studies was undertaken over a full generation to replicate then replace the tainted warrant. For, when human souls, lives, health and well-being are on the line, scientific research becomes particularly subject to ethical considerations.
That’s why we may observe a 2013 paper:
The Ethics of Placebo-controlled Trials: Methodological Justifications
Joseph Millum, Ph.D.a,b and Christine Grady, R.N., Ph.D.a
Abstract
The use of placebo controls in clinical trials remains controversial. Ethical analysis and international ethical guidance permit the use of placebo controls in randomized trials when scientifically indicated in four cases: (1) when there is no proven effective treatment for the condition under study; (2) when withholding treatment poses negligible risks to participants; (3) when there are compelling methodological reasons for using placebo, and withholding treatment does not pose a risk of serious harm to participants; and, more controversially, (4) when there are compelling methodological reasons for using placebo, and the research is intended to develop interventions that can be implemented in the population from which trial participants are drawn, and the trial does not require participants to forgo treatment they would otherwise receive. The concept of methodological reasons is essential to assessing the ethics of placebo controls in these controversial last two cases. This article sets out key considerations relevant to considering whether methodological reasons for a placebo control are compelling.
We instantly see, that placebo control groups are problematic ethically when harm may come to patients because they are given a sugar pill instead of an active drug that is credible enough to be used in the [usually quite expensive trials]. Where, we are not going to be taking up such trials for trivial treatments.
Further to this, there is a sop to the conscience, of having those who give out pills that may be misleadingly labelled, ignorant as to whether the particular pills are active or not. But, there is a record that is being taken. That is, calculated deception and potential for harm are part of the experimental design, an undermining of the fundamental ethical plank of the platform of the medical and nursing profession.
(Mind you, in a day when about a million further victims of the ongoing holocaust of our living posterity in the womb are totted up per week, consciences are doubtless benumbed.)
So, already, there is a general ethical problem, one exacerbated by the principle that to be issued a duly labelled medication by a suitably recognised professional bound under Hippocratic principles, is to have a legitimate expectation of serious treatment. Indeed, that is the foundation of the so-called placebo effect.
In the context of the trials in view regarding drugs for the Covid-19 pandemic, we face a disease that can and too often does seriously harm or kill in a matter of days once symptoms become evident. Further, there is a general challenge to find broadly accepted clinically effective antivirals. However, HCQ and CQ have been noted on record since 2005, as having in vitro effect on a broad range of viruses and as J. Scott Turner reports in Inference Review:
Chloroquine (CQ) and hydroxychloroquine (HCQ) are showing promise as therapeutic treatments for COVID-19 infections. These drugs are widely used for the treatment of malaria, and their use against COVID-19 was a source of some controversy in the early stages of the current pandemic. Nevertheless, the FDA has recently given emergency approval to what had long been a common off-use application for treatment of viral infections. This approval was granted in light of a long history of using CQ and HCQ as antiviral drugs, including during past coronavirus epidemics. Furthermore, informal reports coming from emergency and critical care clinics indicate they are effective against COVID-19, particularly when combined with antiviral agents and antibiotics . . . .
When it came to treating COVID-19, the concern was that this off-use of HCQ posed an unknown risk to patients already at high risk from the viral infection. The debate missed two important points. First, the ad hoc use of CQ and HCQ as antiviral drugs has a history going back nearly forty years, including in the last major coronavirus pandemic of 2002–2003 known as SARS, for Severe Acute Respiratory Syndrome. Second, no one really understands why the quinine family of alkaloids is an effective treatment for malaria.4 For that matter, nobody really understands why CQ and HCQ are effective agents against coronavirus. If this were not enough, CQ and HCQ have also been shown to be effective in a broad spectrum of disorders, including several autoimmune diseases such as lupus and rheumatoid arthritis, diabetes, blood clotting disorders, lymphoma and other blood cancers, and sensory neuropathy. Nobody really understands why CQ and HCQ are effective therapeutic agents for these disorders either.
We have in hand, thousands of cases that support the claim that HCQ (especially in a cocktail) has significant potential to counter Covid-19, and there are several proposed mechanisms, some of which may be acting in concert. One cannot simply dismiss that as though it did not exist. Especially, when we can note the following approval for Dr Raoult’s work:
Research protocol approved by the ANSM and the Île-de-France CPP in progress at the IHU Méditerranée Infection: Treatment of respiratory infections with Coronavirus SARS-Cov2 by hydroxychloroquine Acronym: SARS-CoV2quine.”
Where, further, the following chart appears in his Institute’s site:
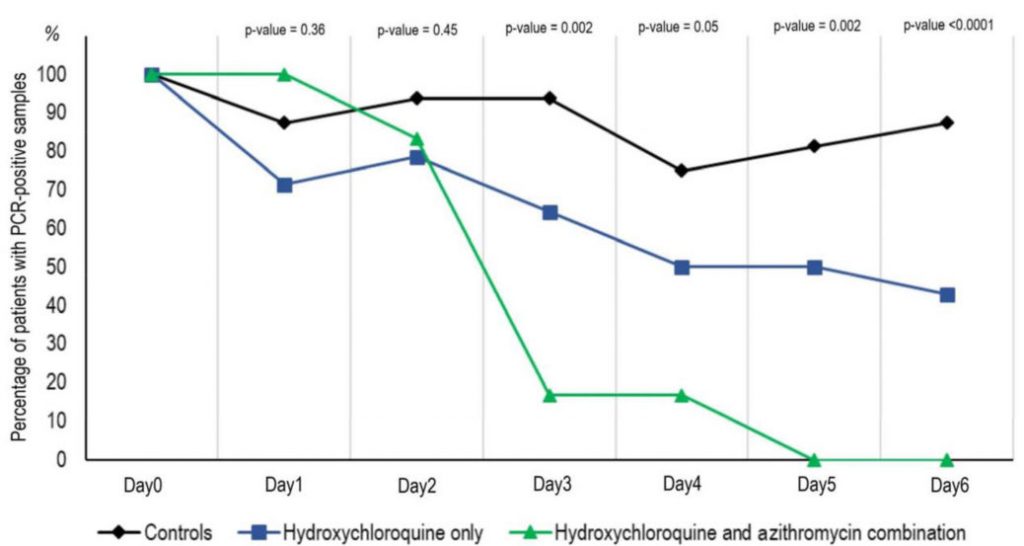
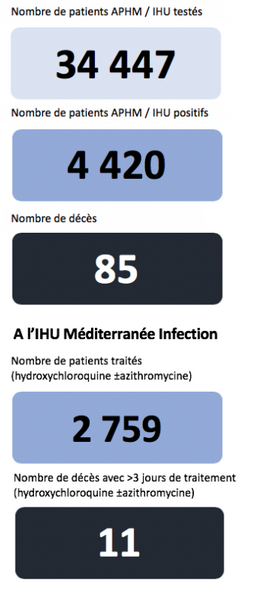
To produce such a chart, of course, considerable experimental work and analysis had to be done. What it shows is a strong reduction in patients with a virus load, implying steady diminution with the Cocktail and less effect with HCL only. That is supported by the onward 80 patient trial and the cumulative 2,759 result:
This seems to document a significant impact on fatality rate and it supports the pattern in the above chart. Where, obviously, we are in a situation of having a respectable number of cases.
Is this result definitive and beyond amendment in light of further results? No, that is never the case for empirical findings. Indeed, strictly, any empirical result could come about by coincidence.
But, that’s not the smart way to bet:
We can observe that we share a common humanity, with fairly similar physiology and biochemistry. Indeed, we overlap with many other life forms. So, if we can find consistent patterns, we are entitled to take them as signs of an underlying mechanism at work. Even, when we do not fully understand it.
In that context, all reasonable evidence counts.
Yes, sometimes a double-blind placebo study is justified (or even if not, has been done). Its results will be best explained on efficacy of a treatment, if successful. But we cannot properly draw a datum line to exclude other empirical evidence, to do so would be to resort to a crooked yardstick.
In our present situation, we do not have the luxury of a year as has been suggested for studies and for vaccines. We have a deadly disease now, a fast-acting one. With a potential treatment by cocktail that seems more effective than HCL alone or other alternatives. Since the drug is a known factor for decades, and as side effects are manageable, it seems that there is warrant to try it, under physician control, and to try it in good enough time to avert complications.
Of course, it is not certain, but this is the strong horse at the moment. Until/unless it is beaten, let us be willing to respect that. END