Food for thought.
U/D: When it reaches the pharmacy . . .
U/D May 19, another Lozano interview:
And, oh yes, breaking 1: Mr Trump is praising — yes, I am NOT using, “touting” — a promising vaccination. Announcement by the firm, here.
Breaking, no 2, courtesy Daily Mail as usual:
 Screenshot from The Daily Mail
Screenshot from The Daily MailOf course, the now standard, it’s risky is in the subheads.
U/D: Video:
Compare our Texas Doctor’s remarks. And then, there is the latest from Dr Raoult:
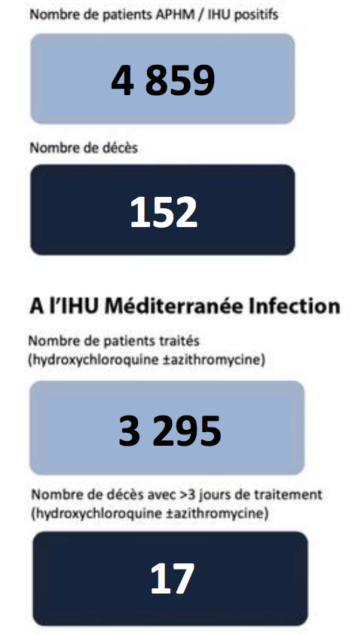
Whose report do you believe, why? END