It’s worth headlining from the CV19 thread:
Jerry, 579: >>Dr. Risch strikes back. https://washex.am/2DFxdij
Hydroxychloroquine works in high-risk patients, and saying otherwise is dangerous
As of Wednesday, some 165,000 people in the United States have died from COVID-19. I have made the case in the American Journal of Epidemiology and in Newsweek that people who have a medical need to be treated can be treated early and successfully with hydroxychloroquine, zinc, and antibiotics such as azithromycin or doxycycline. I have also argued that these drugs are safe and have made that case privately to the Food and Drug Administration.
The pushback has been furious. Dr. Anthony Fauci has implied that I am incompetent, notwithstanding my hundreds of highly regarded, methodologically relevant publications in peer-reviewed scientific literature. A group of my Yale colleagues has publicly intimated that I am a zealot who is perpetrating a dangerous hoax and conspiracy theory. I have been attacked in news articles by journalists who, ignorant of the full picture, have spun hit pieces from cherry-picked sources.
These personal attacks are a dangerous distraction from the real issue of hydroxychloroquine’s effectiveness . . . >>
I picked up, in 632: >> . . . personal attacks are a dangerous distraction from the real issue of hydroxychloroquine’s effectiveness, which is solidly grounded in both substantial evidence and appropriate medical decision-making logic. Much of the evidence is presented in my articles.
[He references: “I have made the case in the American Journal of Epidemiology and in Newsweek that people who have a medical need to be treated can be treated early and successfully with hydroxychloroquine, zinc, and antibiotics such as azithromycin or doxycycline. I have also argued that these drugs are safe and have made that case privately to the Food and Drug Administration.”]
To date, there are no studies whatsoever, published or in pre-print, that provide scientific evidence against the treatment approach for high-risk outpatients that I have described. None. Assertions to the contrary, whether by Fauci, the FDA, or anyone else, are without foundation. They constitute misleading and toxic disinformation.
What do you need to know to evaluate these smears against hydroxychloroquine? The first thing to understand is that COVID-19 has two main stages. [–> see charts here, recall, suppressed by Google, HT Yandex]
I insert, the chart:
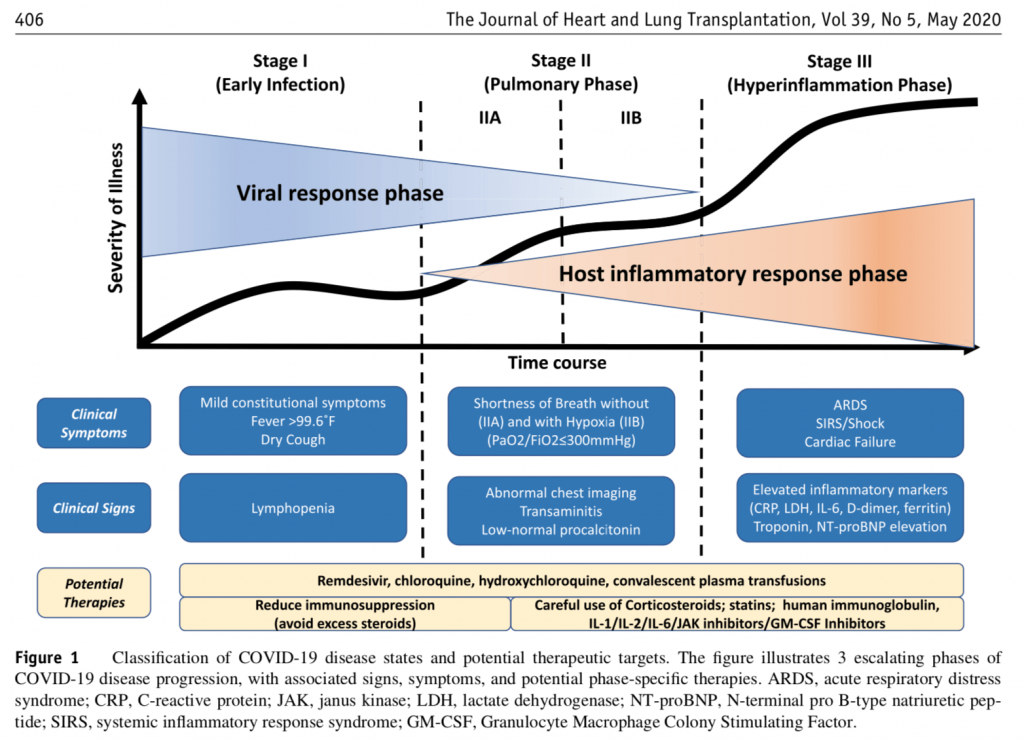
At the first stage, it is a flu-like illness. That illness will not kill you. If you are a high-risk patient and begin treatment immediately, you will almost certainly be done with it in a few days. When not [adequately?] treated, high-risk patients may progress. The virus then causes severe pneumonia and attacks many organs, including the heart. In this second stage, hydroxychloroquine is not effective.
So, if you are told that hydroxychloroquine doesn’t work, ask this question: In which patients? Does it not work in those who have just started to have symptoms, or those sick enough to require hospitalization?
The second thing to know is that most low-risk patients survive without treatment. Low risk means you are under age 60 and have no chronic conditions such as diabetes, obesity, and hypertension, have no past treatment for cancer, are not immunocompromised, etc. High risk means you are over 60 or you have one or more of those chronic conditions. High-risk patients need immediate treatment when they first show symptoms. One should not wait for the COVID-19 test result, which can take days and can be wrong. Again, when Fauci and others say that randomized controlled trials show no benefit for hydroxychloroquine, you must ask: In which group of patients?
Every randomized controlled trial to date that has looked at early outpatient treatment has involved low-risk patients, patients who are not generally treated. In these studies, so few untreated control patients have required hospitalization that significant differences were not found. There has been only one exception: In a study done in Spain with low-risk patients, a small number of high-risk nursing home patients were included. For those patients, the medications cut the risk of a bad outcome in half.
I reiterate: If doctors, including any of my Yale colleagues, tell you that scientific data show that hydroxychloroquine does not work in outpatients, they are revealing that they can’t tell the difference between low-risk patients who are not generally treated and high-risk patients who need to be treated as quickly as possible. Doctors who do not understand this difference should not be treating COVID-19 patients.
What about medication safety? On July 1, the FDA posted a “black-letter warning” cautioning against using hydroxychloroquine “outside of the hospital setting,” meaning in outpatients. But on its website just below this warning, the FDA stated that the warning was based on data from hospitalized patients. To generalize and compare severely ill patients with COVID-induced pneumonia and possibly heart problems to outpatients is entirely improper.
In fact, the FDA has no information about adverse events in early outpatient use of hydroxychloroquine. The only available systematic information about adverse events among outpatients is discussed in my article in the American Journal of Epidemiology, where I show that hydroxychloroquine has been extremely safe in more than a million users.
It is a serious and unconscionable mistake that the FDA has used inpatient data to block emergency use petitions for outpatient use. Further, already back in March, the FDA approved the emergency use of hydroxychloroquine for hospitalized patients, for whom it is demonstrably less effective than for outpatients. If hydroxychloroquine satisfied the FDA criteria for emergency inpatient use in March, it should more than satisfy those criteria now for outpatient use, where the evidence is much stronger.>>
Some reconsideration may be in order. END
PS: As a part of that, I have consistently pointed to the tendency to insist on the “need” for placebo controlled studies (an issue Risch also spoke to in his Newsweek article). In the discussion, I took time to address a bit of algebra:
KF, 566: >> [Let me insert a diagram on sustainability oriented decision making, adapted from the Bariloche Foundation of Argentina:]
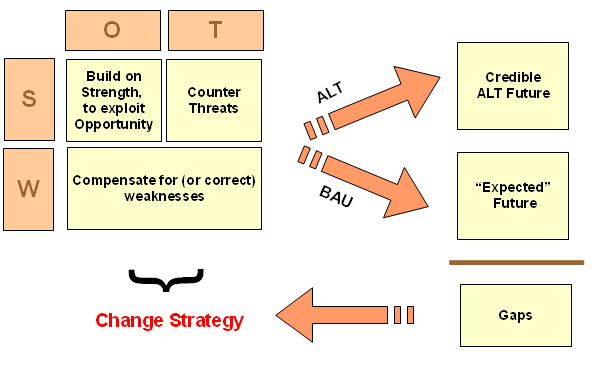
7: Where, BAU is in fact a natural baseline of reference. We seek a more satisfactory alternative, ALT. It must be credible enough incrementally to justify onward exploration and that first requires becoming a candidate for more costly investigation that shifts epistemic probabilities. Where, of arguments by/among clever people there is no end, so empirical demonstration at various levels is pivotal.
8: Here, epistemology of empirically based knowledge does not allow for gold standards that impose selective hyperskepticism against otherwise reasonable evidence. Evidence is evidence (and various uncertainties, risks and potential for errors cannot be wholly eliminated). So, we must recognise that BAU is a baseline/ benchmark/ control, and there is no strict necessity to construct an artificial, no effective treatment baseline; call it 0TB.
9: After all, the point is really to improve outcomes from BAU, and gap analysis ALT vs BAU has no inherent reference to 0TB. Algebraically, on credible or observed outcomes,
(ALT – 0TB) – (BAU – 0TB) = ALT – BAU
Where, with people as test subjects, if 0TB is based on deception and has potential for significant harm, it becomes ethically questionable. We know of extreme cases of concentration camp experimentation, the Tuskegee syphilis atrocity and more. However in more recent times, people have been subjected to fake surgeries under general anesthesia etc. The placebo effect has covered a multitude of sins.
10: In the face of pandemic, urgency is another issue. What yields results in a timeframe relevant to taming the surge of cases becomes a highly relevant criterion. As does the tradeoff of lives lost under various treatment, public health [e.g. quarantines vs general lockdown] and policy options. Where, relevantly, economic dislocation carries a toll in health and lives too. (It is suggested by some that deaths of despair and from postponed medical procedures may/do exceed those attributed to the epidemic.) This means BTW that the dismal science, Economics, has a seat at the decision makers’ table as of right.>>
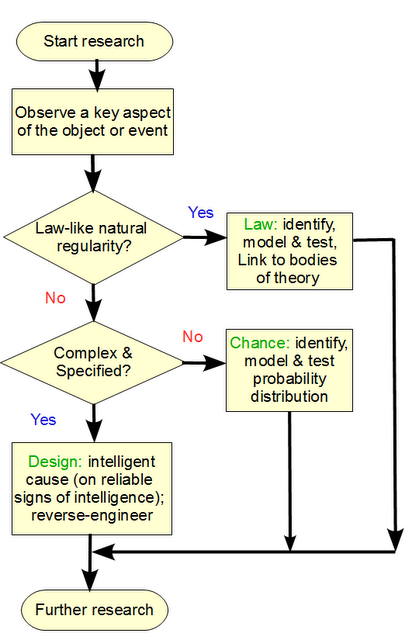
PPS: Design inference explanatory filter, per aspect form:
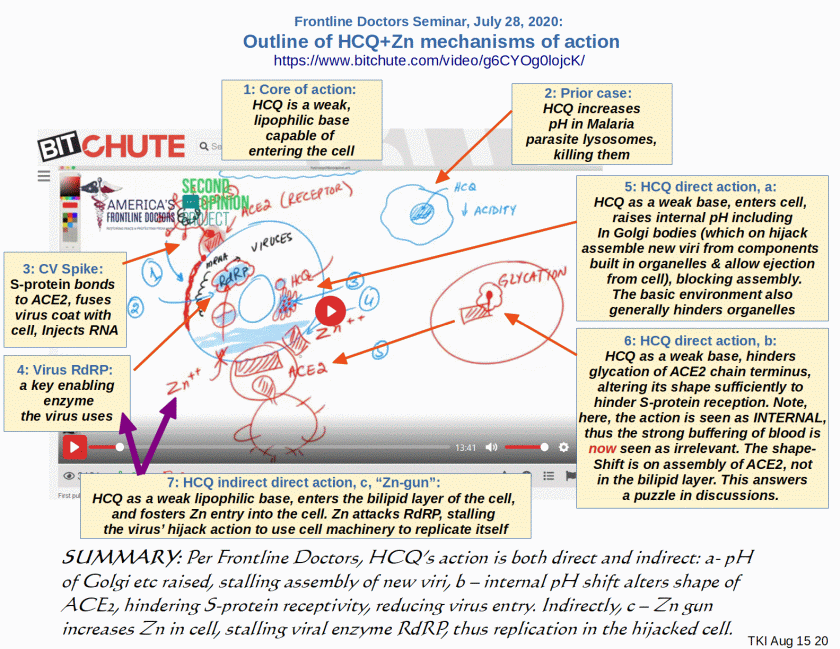
PPPS: The HCQ mechanisms, summarised by Frontline Doctors: