One of the key steps in dismissing evidence of efficacy of hydroxychloroquine-based cocktails in treating early stageCovid-19 for patients in vulnerable groups on an outpatient basis is the use of the premise that such evidence is of low quality as it does not match the “gold standard” of placebo-controlled, randomised tests (often. RCT’s). However, observations are observations, natural regularities are often observable from the first few trials, evidence is evidence, ethical and practical considerations are real, and valid scientific methods do not reduce to applied statistics.
It is in that context that we should attend carefully to remarks by Dr Thomas Frieden, writing in NEJM 3 1/2 years ago, in terms that uncannily anticipate our current woes:
Despite their strengths, RCTs have substantial limitations. Although they can have strong internal validity, RCTs sometimes lack external validity; generalizations of findings outside the study population may be invalid.2,4,6 RCTs usually do not have sufficient study periods or population sizes to assess duration of treatment effect (e.g., waning immunity of vaccines) or to identify rare but serious adverse effects of treatment, which often become evident during postmarketing surveillance and long-term follow-up but could not be practically assessed in an RCT. The increasingly high costs and time constraints of RCTs can also lead to reliance on surrogate markers that may not correlate well with the outcome of interest. Selection of high-risk groups increases the likelihood of having adequate numbers of end points, but these groups may not be relevant to the broader target populations. These limitations and the fact that RCTs often take years to plan, implement, and analyze reduce the ability of RCTs to keep pace with clinical innovations; new products and standards of care are often developed before earlier models complete evaluation. These limitations also affect the use of RCTs for urgent health issues, such as infectious disease outbreaks, for which public health decisions must be made quickly on the basis of limited and often imperfect available data. RCTs are also limited in their ability to assess the individualized effect of treatment, as can result from differences in surgical techniques, and are generally impractical for rare diseases.
Many other data sources can provide valid evidence for clinical and public health action. Observational studies, including assessments of results from the implementation of new programs and policies, remain the foremost source, but other examples include analysis of aggregate clinical or epidemiologic data . . .
He also presents a table of options with strengths and weaknesses, which we now sample:
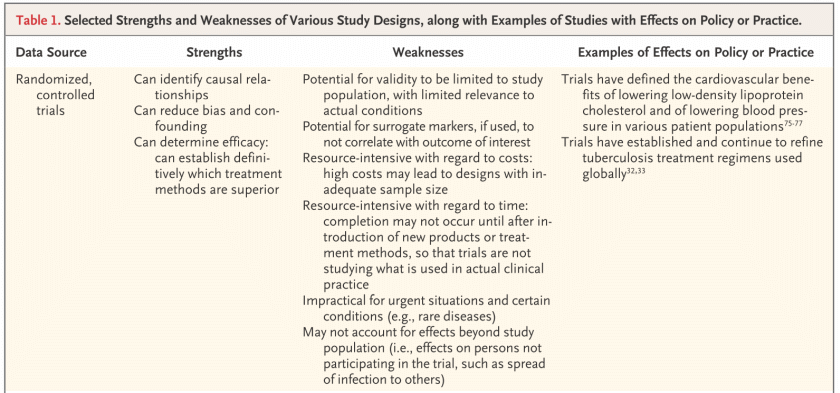
Later in the same article, as he concludes, he also notes:
There is no single, best approach to the study of health interventions; clinical and public health decisions are almost always made with imperfect data (Table 1). Promoting transparency in study methods, ensuring standardized data collection for key outcomes, and using new approaches to improve data synthesis are critical steps in the interpretation of findings and in the identification of data for action, and it must be recognized that conclusions may change over time. There will always be an argument for more research and for better data, but waiting for more data is often an implicit decision not to act or to act on the basis of past practice rather than best available evidence. The goal must be actionable data — data that are sufficient for clinical and public health action that have been derived openly and objectively and that enable us to say, “Here’s what we recommend and why.”
In that context, it is appropriate for me to again highlight a diagram on sustainability oriented decision making, adapted from the Bariloche Foundation of Argentina:
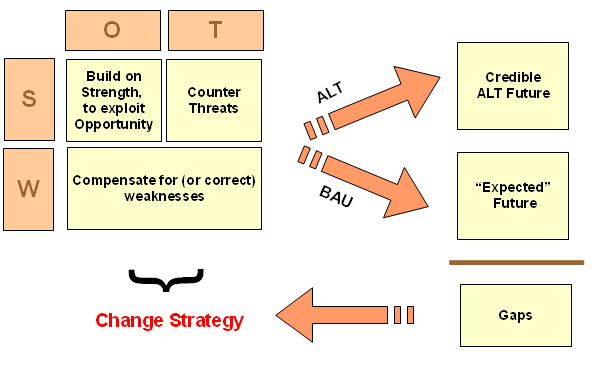
Where, BAU is in fact a natural baseline of reference. We seek a more satisfactory alternative, ALT. It must be credible enough incrementally to justify onward exploration and that first requires becoming a candidate for more costly investigation that shifts epistemic probabilities. Where, of arguments by/among clever people there is no end, so empirical demonstration at various levels is pivotal.
Here, epistemology of empirically based knowledge does not allow for gold standards that impose selective hyperskepticism against otherwise reasonable evidence. Evidence is evidence (and various uncertainties, risks and potential for errors cannot be wholly eliminated). So, we must recognise that BAU is a baseline/ benchmark/ control, and there is no strict necessity to construct an artificial, no effective treatment baseline; call it 0TB.
After all, the point is really to improve outcomes from BAU, and gap analysis ALT vs BAU has no inherent reference to 0TB. Algebraically, on credible or observed outcomes, we see this from
(ALT – 0TB) – (BAU – 0TB) = ALT – BAU
Where, with people as test subjects, if 0TB is based on deception — e.g. sugar pills deliberately mislabelled and presented under false colours and ceremonies of medicine and research in the face of significant risk of harm to vulnerable patients — and has potential for significant harm, it becomes ethically questionable. We know of extreme cases of concentration camp experimentation, the Tuskegee syphilis atrocity and more. However in more recent times, people have been subjected to fake surgeries under general anesthesia etc. The placebo effect has covered a multitude of sins.
In the face of pandemic, urgency is another issue. What yields results in a timeframe relevant to taming the surge of cases becomes a highly relevant criterion. As does the tradeoff of lives lost under various treatment, public health [e.g. quarantines vs general lockdown] and policy options. Where, relevantly, economic dislocation carries a toll in health and lives too. (It is suggested by some that deaths of despair and from postponed medical procedures may/do exceed those attributed to the epidemic.) This means BTW that the dismal science, Economics, has a seat at the decision makers’ table as of right.
It is time for mindset change. END
PS/UD Aug 15: I have found at Bit Chute, a July 28 Frontline Doctors seminar which describes several mechanisms of action. Accordingly, I take liberty to annotate a screenshot, summarising several mechanisms of action described by these Doctors [cf. here for their references], but which are hard to find because of now almost pervasive censorship:
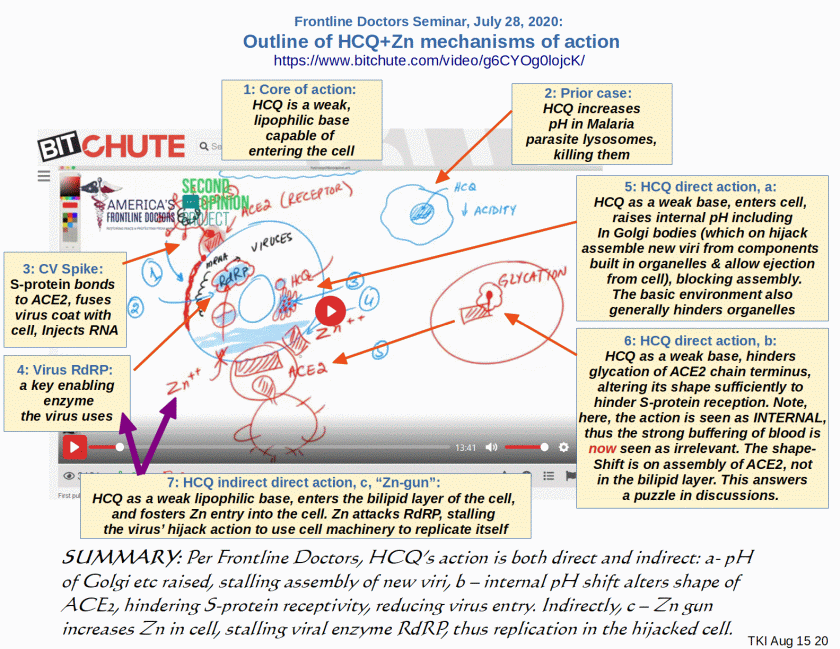
I note, this first answers a puzzle on the mode of action, shape-shift of ACE2: the shift is INTERNAL to the cell by hindering “glycation” of the final AA (thus prior to exposure to buffering of blood etc), altering the shape enough to hamper S-protein reception. This reduces fusion with bilipid layer and RNA injection.
Other direct mechanisms as noted, reduce intracellular acidity thus action of organelles. They highlight stalling of assembly of new viri in the Golgi bodies, with implication of blocking export of fresh viri, thus hampering the multiplication chain. The by now well known indirect activity is that as a lipophilic molecule, HCQ enters the cell bilipid layer membrane, acting as a Zn ionophore, i.e. it “shoots” Zn into the cell. Zn in turn hinders a key viral enzyme, RdRP.
Thus, we see a plausible picture of causal action, involving multiple, synergistic effects. This lends credibility to the use of HCQ-based cosctails in treating the early viral phases of CV19.
PPS: Given tendencies to be dismissive, I here reproduce two key illustrations/charts from Dr Raoult’s work on now over 3,000 patients at IHU in Marseilles France.
First, his statistical summary on difference made with vulnerable groups through HCQ-Azithromycin treatment, by May:
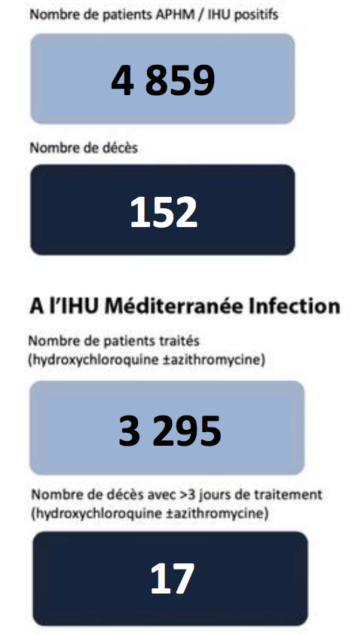
We note that expected death rates for vulnerable groups are as high as 15%.
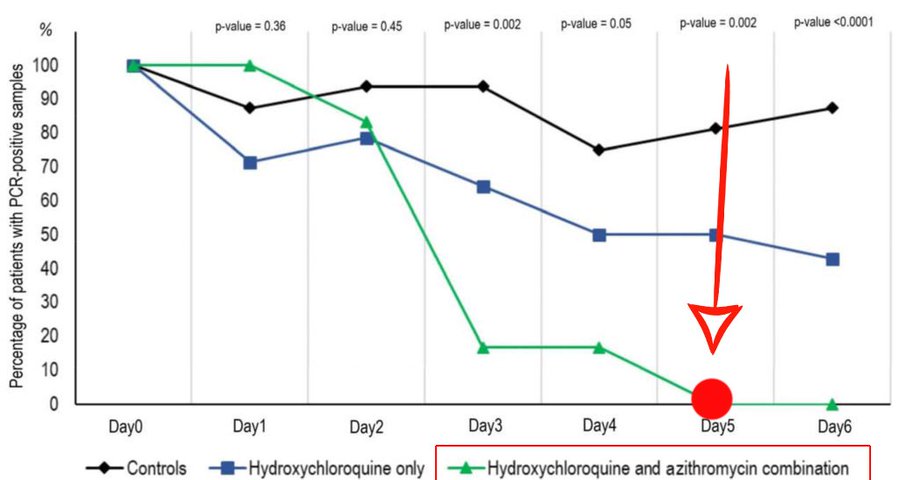
Next, here is a chart from his early, about 80 patient stage, illustrating rapid reduction of viral load . . . for which we now have specific, scientifically plausible causal mechanisms on the table:
I add, just for stirring the pot, a Frontline Doctors chart on CV19 case fatality rates vs accessibility of HCQ:
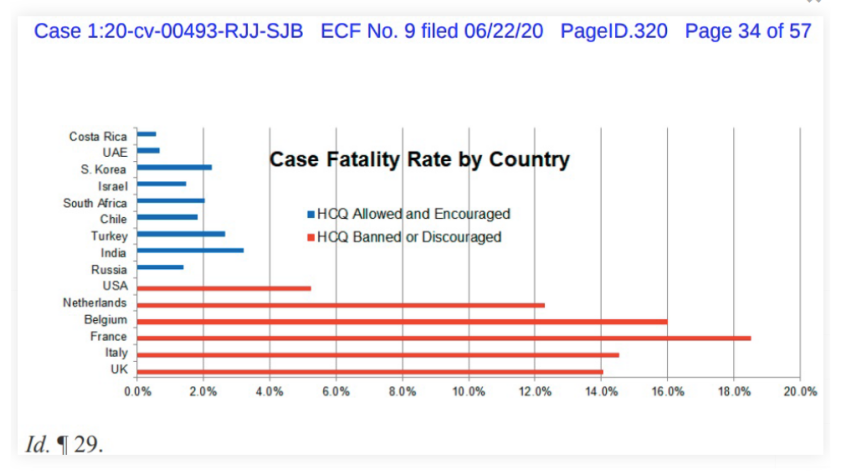
Thus, we see good reason to accept that HCQ-based cocktails (which were available from the outset of the pandemic) are credibly effective and should not have been treated with the extreme skepticism, hostility and suppression we have instead seen. Note, the leader of the Frontline Doctors, Dr Simone Gold, was fired immediately on leading a public protest. Frankly, that smacks of whistleblower retaliation.
It is appropriate to raise pointed questions, through the voice of the doctors writing an open letter to Dr Fauci:
>>There is currently no recommended pharmacologic early outpatient treatment for individuals in the flu stage of the illness, correct?
It is true that COVID-19 is much more lethal than the flu for high-risk individuals such as older patients and those with significant comorbidities, correct?
Individuals with signs of early COVID-19 infection typically have a runny nose, fever, cough, shortness of breath, loss of smell, etc., and physicians send them home to rest, eat chicken soup etc., but offer no specific, targeted medications, correct?
These high-risk individuals are at high risk of death, on the order of 15% or higher, correct?
So just so we are clear—the current standard of care now is to send clinically stable symptomatic patients home, “with a wait and see” approach?
Are you aware that physicians are successfully using Hydroxychloroquine combined with Zinc and Azithromycin as a “cocktail” for early outpatient treatment of symptomatic, high-risk, individuals?
Have you heard of the “Zelenko Protocol,” for treating high-risk patients with COVID 19 as an outpatient?
Have you read Dr. Risch’s article in the American Journal of Epidemiology of the early outpatient treatment of COVID-19?
Are you aware that physicians using the medication combination or “cocktail” recommend use within the first 5 to 7 days of the onset of symptoms, before the illness impacts the lungs, or cytokine storm evolves?
Again, to be clear, your recommendation is no pharmacologic treatment as an outpatient for the flu—like symptoms in patients that are stable, regardless of their risk factors, correct?
Would you advocate for early pharmacologic outpatient treatment of symptomatic COVID-19 patients if you were confident that it was beneficial?
Are you aware that there are hundreds of physicians in the United States and thousands across the globe who have had dramatic success treating high-risk individuals as outpatients with this “cocktail?”
Are you aware that there are at least 10 studies demonstrating the efficacy of early outpatient treatment with the Hydroxychloroquine cocktail for high-risk patients — so this is beyond anecdotal, correct?
If one of your loved ones had diabetes or asthma, or any potentially complicating comorbidity, and tested positive for COVID-19, would you recommend “wait and see how they do” and go to the hospital if symptoms progress?
Even with multiple studies documenting remarkable outpatient efficacy and safety of the Hydroxychloroquine “cocktail,” you believe the risks of the medication combination outweigh the benefits?
Is it true that with regard to Hydroxychloroquine and treatment of COVID-19 infection, you have said repeatedly that “The Overwhelming Evidence of Properly Conducted Randomized Clinical Trials Indicate No Therapeutic Efficacy of Hydroxychloroquine (HCQ)?”
But NONE of the randomized controlled trials to which you refer were done in the first 5 to 7 days after the onset of symptoms- correct?
All of the randomized controlled trials to which you refer were done on hospitalized patients, correct?
Hospitalized patients are typically sicker that outpatients, correct?
None of the randomized controlled trials to which you refer used the full cocktail consisting of Hydroxychloroquine, Zinc, and Azithromycin, correct?
While the University of Minnesota study is referred to as disproving the cocktail, the meds were not given within the first 5 to 7 days of illness, the test group was not high risk (death rates were 3%), and no zinc was given, correct?
Again, for clarity, the trials upon which you base your opinion regarding the efficacy of Hydroxychloroquine, assessed neither the full cocktail (to include Zinc + Azithromycin or doxycycline) nor administered treatment within the first 5 to 7 days of symptoms, nor focused on the high-risk group, correct?
Therefore, you have no basis to conclude that the Hydroxychloroquine cocktail when used early in the outpatient setting, within the first 5 to 7 days of symptoms, in high risk patients, is not effective, correct?
It is thus false and misleading to say that the effective and safe use of Hydroxychloroquine, Zinc, and Azithromycin has been “debunked,” correct? How could it be “debunked” if there is not a single study that contradicts its use?
Should it not be an absolute priority for the NIH and CDC to look at ways to treat Americans with symptomatic COVID-19 infections early to prevent disease progression?
The SARS-CoV-2/COVID-19 virus is an RNA virus. It is well-established that Zinc interferes with RNA viral replication, correct?
Moreover, is it not true that hydroxychloroquine facilitates the entry of zinc into the cell, is a “ionophore,” correct?
Isn’t also it true that Azithromycin has established anti-viral properties?
Are you aware of the paper from Baylor by Dr. McCullough et. al. describing established mechanisms by which the components of the “HCQ cocktail” exert anti-viral effects?
So- the use of hydroxychloroquine, azithromycin (or doxycycline) and zinc, the “HCQ cocktail,” is based on science, correct?>>