Elaborated Tunnel Architectures in Enzyme Systems point to a designed setup
RNA and DNA belong to the four basic building blocks of life. They are complex macromolecules made of three constituents: the base, the backbone, which is the ribose five-carbon sugar, and phosphate, the moiety which permits DNA polymerization and catenation of monomers, to become polymers. The nucleobases are divided into pyrimidine and purines. These bases must be made in complex biosynthesis pathways in the cell, requiring several molecular machines, and enzymes, that perform the gradual, stepwise operations to yield the nucleobases, which, in the end, are handed over for further processing. Pyrimidines, one of the two classes, require 7 enzymes, of which Carbamoyl phosphate synthase II is the first in the production line.
In bacteria, a single enzyme supplies carbamoyl phosphate for the synthesis of arginine and pyrimidines. The bacterial enzyme has three separate active sites, spaced along a tunnel nearly 100 Å long. Bacterial carbamoyl phosphate synthetase provides a vivid illustration of the channeling of unstable reaction intermediates between active sites. This reaction consumes two molecules of ATP: One provides a phosphate group and the other energizes the reaction. The need for this channel exists to efficiently translocate reactive gaseous molecules that can either be toxic to the cell or are reactive intermediates that need to be delivered to complete a coupled reaction.
Comment: Consider that no lifeform exists that does not use DNA and RNA. Therefore, the synthesis of these molecules is a prerequisite for life. The origin of this metabolic pathway can therefore not be explained through evolution. Either it was design or random nonguided fortunate events.
Tunnel Architectures in Enzyme Systems that Transport Gaseous Substrates
Derinkuyu Underground City in Cappadocia, Turkey, is one of the deepest and most fascinating multilevel subterranean cities, excavated in tunnel systems. Specifically constructed, elaborated Air ducts ensure fresh oxygen supply, and the oxygen ratio inside never changes no matter at what level one is in. Such systems are always engineering marvels, and must be precisely calculated, and constructed. Remarkably, some proteins act similarly and exist in molecular biological systems.
Ruchi Anand (2021): Tunnels connect the protein surface to the active site or one active site with the others and serve as conduits for the convenient delivery of molecules. Tunnels transferring small molecules such as N2, CH4, C2H6, O2, CO, NH3, H2, C2H2, NO, and CO2 are termed gaseous tunnels. Conduits that have a surface-accessible connection and can accept gases from the surroundings are named external gaseous (EG) tunnels. Whereas, buried gaseous tunnels that do not emerge to the surface are named internal gaseous (IG) tunnels. In some cases, the tunnels can be performed, permanently visible within the protein structure such that the natural breathing motions in proteins do not alter the tunnel dimensions to the extent that the radius of the gaseous tunnel falls below the minimum threshold diameter, e.g., carbamoyl phosphate synthetase (CPS) has a preformed tunnel. In contrast, it can be transient such that the tunnel diameter is not sufficiently wide enough to allow the incoming molecule to pass through it or certain constrictions in the tunnel block its delivery. This could be either to control the frequency of molecules traveling across or to coordinate and facilitate coupled reaction rates. Another possible scenario of transient tunnel formation is one in which the tunnel is nonexistent in the apo state, and only upon significant conformational change, under appropriate cues, is the tunnel formed. In several cases transient tunnels require intermediate/substrate-induced conformational changes in the tunnel residues to open up for the transport of the incoming molecule, within the respective enzyme. These tunnels undergo enormous fluctuations and switch between open and close states. It is remarkable that the presence of these conduits, which are as long as 20−30 Å and even longer like 96 Å in CPS,6a run inside the protein body, forming pores that serve as highways for transport of these gaseous molecules. In several cases, an added level of tuning into the tunnel architecture is introduced by incorporating gating mechanisms into the EG and IG tunnel architectures.
Gates serve as checkpoints and vary from system to system; some are as simple as an amino acid blocking the path which moves out upon receiving appropriate cues such as the swinging door type in cytidine triphosphate synthase (CTP) and in others more complex arrangement of amino acids come together to form control units such as aperture gates, drawbridge, and shell type gates. These tunnels and their gates are connected via an active communication network that spans between distal centers and hence introduces both conformation and dynamic allostery into the protein systems. It is not uncommon to observe long-distance allosteric networks that can be dynamic in nature and transiently formed via the motion of loop elements, secondary structural rearrangements, or of entire domains.
EXTERNAL GASEOUS (EG) TUNNEL ARCHITECTURES
EG tunnels connect the bulk solvent with the active site of an enzyme. These tunnels are found in several enzymes that accept gaseous substrates to facilitate their delivery to the buried active site. A class of predominant gaseous substrates are alkanes such as methane and ethane gases that are oxidized aerobically or via anaerobic pathways. Recently, the crystal structure of the enzyme that anaerobically oxidizes ethane to ethylCoM from Candidatus Ethanoperedens thermophilum was determined, and named it ethylCoM reductase. The enzyme belongs to the broad methylCoM reductase superfamily, which oxidizes methane. The ethylCoM reductase has a 33 Å tunnel that runs across the length of the protein. Interestingly, the EG tunnel present in ethylCoM reductase has some very unique features. At the end of the tunnel, near the Ni-cofactor F430 active site, there are several residues that are post-translationally modified. Methylated amino acids, such as S-methylcysteine, 3-methylisoleucine, 2(S)-methylglutamine, and N2 -methylhistidine line the tunnel. It is likely that these residues tune the enzyme to select for ethane by creating a very hydrophobic environment and prevent similar-sized hydrophilic molecules such as methanol from reaching the active center. The larger hydrophobic alkanes are selected out via optimization of the tunnel diameter, which is fit to accommodate ethane. Another example of an alkane transporting tunnel exists in soluble methane monooxygenase (sMMO) that performs C− H functionalization by breaking the strongest C−H bond, among saturated hydrocarbons, in methane and aerobically oxidizes it to form methanol. In methanotrophs, these enzymes are tightly regulated, and the complex formation between the two proteins, hydroxylase MMOH and regulatory protein MMOB, is required for function. The EG tunnel formed in this system is very hydrophobic, and the diameter is such that it only allows for smaller gases such as methane and O2 to percolate into the di-Fe cluster harboring active site. In Methylosinus trichosporium OB3b, half of the tunnel is at the interface of the MMOH/MMOB complex, and another half of the tunnel is buried within MMOH, where the oxidation reaction is catalyzed. As an added control feature, the complex has multiple gates to regulate its function. Residues W308 and P215 guard the entrance of the substrate molecules and block the formation of the EG tunnel in the absence of the complex between MMOH and MMOB.
Comment: This demonstrates and exemplifies how in many cases, single monomers have important functions, and changing them through mutations can remove the function of the entirety of the enzyme.
Upon complexation, a conformational change is triggered, and these residues move out of the path, opening the passage for the entire tunnel. When the upper gating residues move upon MMOB/MMOH complex formation, another residue F282 right near the active site also concomitantly undergoes a shift, allowing methane and oxygen to access the di-Fe center. MMOH also has an alternative secondary hydrophilic passage, accessible only when MMOB/MMOH complex dissociates which allows the polar methanol product to be released through it. The gating residues, F282 in the hydrophobic EG tunnel and E240 in the hydrophilic passage, switch between open and close states alternately upon binding/unbinding of MMOB and hence opens one of the two tunnels at a time. This regulates the flow of substrates and products and avoids overoxidation of methanol by releasing it through the hydrophilic passage prior to the entry of substrates in the active site via the hydrophobic EG tunnel.
One of the most common gaseous substrates for which several examples of tunneling enzymes exist is oxygen (O2). It is used in several important oxidation reactions for the generation of essential pathway intermediates and also is a key transport gas in cells. Interestingly in several cases, oxygen is transported to the desired site via molecular tunnels, perhaps to modulate its flow. There are two types of tunnel architectures that are prevalent: first, where there is a main tunnel connected to several subsidiary tunnels, and second, those with fewer tunnels but with stringent gating controls. For instance, soybean lipoxygenase-1 is an example of a multitunnel system that has eight EG tunnels, out of which the one that is formed by hydrophobic residues, such as L496, I553, I547, and V564, has the highest throughput and is identified as the main gaseous tunnel for delivering O2 to the reaction center. It catalyzes the stereospecific peroxidation of linoleic acid via forming a pentadienyl radical intermediate. Under oxygen-deficient conditions, the intermediate escapes from the active site to the bulk and forms four products, i.e., 13S-, 13R-, 9S-, and 9R-hydroperoxy-octadecadienoic acid, in equal distributions. However, under ambient O2 conditions, the EG tunnel delivers O2 efficiently into the active site which has a properly positioned and oriented radical intermediate. Here, O2 is delivered by the EG tunnel such that it stereo- and regiospecifically attacks the radical intermediate to yield 13S-hydroperoxy-octadecadienoic acid as a major product with ∼90% yield. It has also been shown that when the EG tunnel residue L496 is mutated to a bulky tryptophan, it opens up a new gaseous tunnel for O2 delivery, where it attacks at the different side of the pentadienyl intermediate, preferring the formation of 9S- and 9Rproducts. This example showed the importance of the gaseous tunnel in determining the stereo- and regiospecificity for product formation
INTERNAL GASEOUS (IG) TUNNEL ARCHITECTURES
While the EG tunnels transport gases and have pores that are accessible to the surface, there is another class of tunnels formed within the core of the enzyme system, buried in the body of the protein, called the IG tunnels.
Question: How could these tunnels be the product of evolutionary pressures, requiring long periods of time, if, in case the tunnel that protects the toxic intermediates is not instantiated from the beginning, the products would leak, and eventually kill the cell? This is an all-or-nothing business, where these tunnels had to be created right from the start, fully set up and developed.
These systems generally have the tunnel connecting two reactive centers, and the product of one reaction is transported to the second active site. In some cases, an IG tunnel network, instead of leading to another active site, can also lead to the lipid membrane so as to directly access the active site of membrane-bound enzymes. The substrate is generated within one of the active centers and is in the limiting amount as well as it could be toxic or unstable in the presented environment. Therefore, to ensure it reaches the destination reaction center, nature has devised strategies by constructing IG tunnels which, in several instances, are transient tunnels that only form upon entry of substates and have much more controlled and complex gating architectures. 57
Comment: This is truly fascinating evidence of intended design for important functions: To direct gases to where they are needed to perform a reaction.
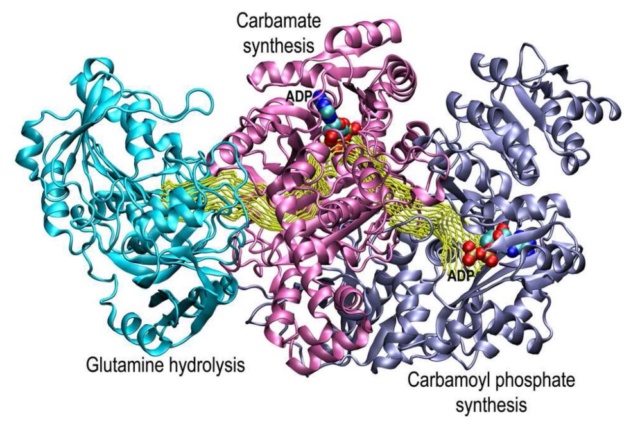
Image description: The structure of carbamoyl phosphate synthetase
The small subunit that contains the active site for the hydrolysis of glutamine is shown in green. The N-terminal domain of the large subunit that contains the active site for the synthesis of carboxy phosphate and carbamate is shown in red. The C-terminal domain of the large subunit that contains the active site for the synthesis of carbamoyl phosphate is shown in blue. The two molecular tunnels for the translocation of ammonia and carbamate are shown in yellow dotted lines 56
Nucleotide metabolism: By evolution?
G. Caetano-Anollés (2013): The origin of metabolism has been linked to abiotic chemistries that existed in our planet at the beginning of life. While plausible chemical pathways have been proposed, including the synthesis of nucleobases, ribose and ribonucleotides, the cooption of these reactions by modern enzymes remains shrouded in mystery. Pathways of nucleotide biosynthesis, catabolism, and salvage originated ∼300 million years later byconcerted enzymatic recruitments and gradual replacement of abiotic chemistries. The simultaneous appearance of purine biosynthesis and the ribosome probably fulfilled the expanding matter-energy and processing needs of genomic information. 59
Comment: These are assertions, clearly not based on scientific data and observations, but ad-hoc conclusions that lack evidence.
56. Yubo Fan: A Combined Theoretical and Experimental Study of the Ammonia Tunnel in Carbamoyl Phosphate Synthetase 2009
57. Ruchi Anand: Tunnel Architectures in Enzyme Systems that Transport Gaseous Substrates December 3, 2021
58. Sérgio M. Marques: Role of tunnels, channels and gates in enzymatic catalysis 2016
59. Gustavo Caetano-Anollés: Structural Phylogenomics Reveals Gradual Evolutionary Replacement of Abiotic Chemistries by Protein Enzymes in Purine Metabolism March 13, 2013