This video summarises a direct, molten salt based electro-reduction process for metals, especially Titanium:
(Titanium, of course, is a rather abundant but hard to win “super-metal.” See Wiki here for a more detailed summary. The process extends to other metals and of course turns on having abundant electrical energy.)
Let me add an illustration of the electrolytic cell:
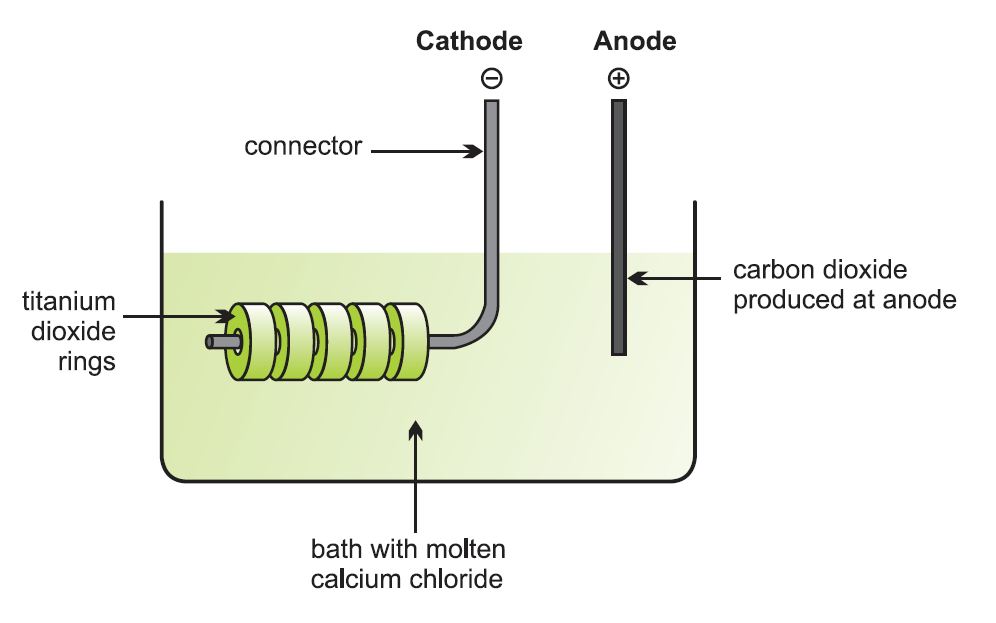
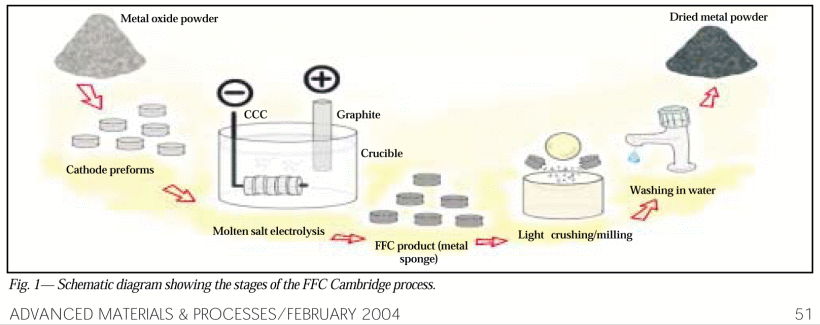
. . . with a broader overview, 2004:
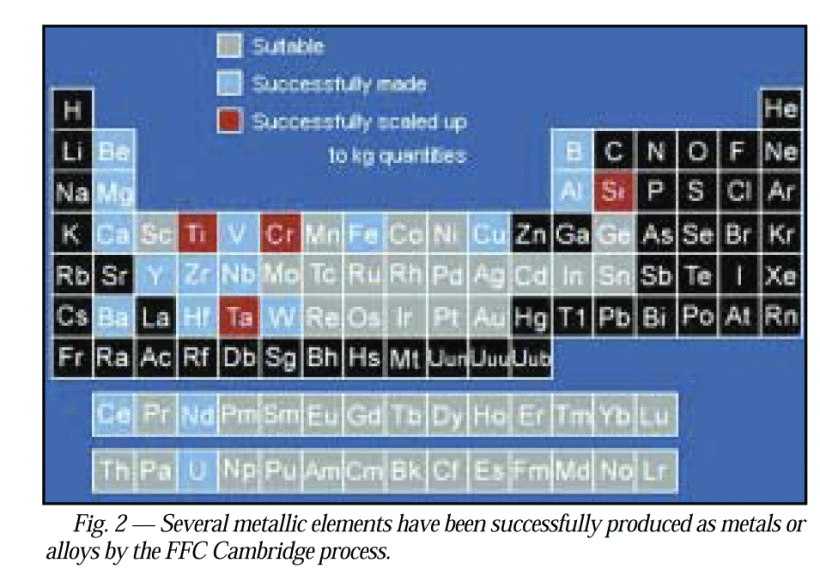
. . . nb here on universality, pardon the resolution, red — already in kg q’ties by 2004, blue achieved, grey, suitable . . . observe esp. not only Fe, Al etc but Si, Ge, Ga [not As though], W [= Tungsten, aka Wolfram], U, Th, Pu, as well as the rare earths that are key to high performance magnets thus motors etc:
. . . also, a comparison with Kroll clipped from the vid, illustrating relative simplicity and suggesting reduced financial and energy costs:

It’s time to start thinking about taking our civilisation forward, and metals are a critical material base. Of course, a natural target for energy production is advanced nuclear technologies. DV, soon — but first let us ponder the idea of open source re-industrialisation as a kick-starter for development and tech base for solar system colonisation. Why have we had a 50-year pause, near enough, after first landing men on the Moon? END